Research Feature of the Month
Each month, we spotlight a standout publication by our faculty that exemplifies the innovation and impact of MSE research. From advancing sustainable materials to breakthrough applications in healthcare and energy, these works reflect our commitment to pushing the frontiers of materials science.
Oct 2025
Next-gen aluminium-ion batteries made possible with lean-water hydrogels
This feature was prepared in collaboration with NTU MSE's Prof. Alex Yao and PhD Student, Liew Jin Jie.
As society accelerates toward sustainable energy, lithium-ion batteries continue to dominate energy storage for electric vehicles and portable electronics. Yet they face severe limitations – lithium extraction has a high environmental cost, the electrolytes used in these batteries can pose safety risks, and the batteries are sensitive to temperature changes.
Materials scientists are now exploring next-generation batteries made from more abundant, safer metals such as aluminium. The metal, if successfully developed into a battery, boasts a high theoretical capacity and natural stability, while being both safer and easier to extract than lithium.
However, aqueous aluminium-ion batteries (AAIBs) — which use water-based electrolytes for safety — often fail because water reacts too aggressively with aluminium. Previous attempts to solve this problem relied on “water-in-salt” electrolytes, organic solvents, or chemical additives to curb water’s reactivity, which were often costly, complex, or environmentally unfriendly.
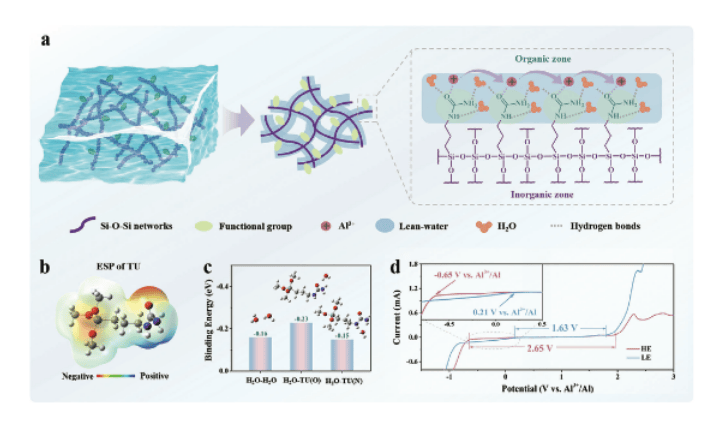
Scientists at NTU MSE set out to design a suitable electrolyte that is low-cost, non-toxic, easy to scale up, and high in leak resistance and flexibility, while controlling water reactivity. Their attention eventually turned to hydrogel electrolytes — materials that trap water within a solid-like network — with exceptional physicochemical and electrochemical properties.
The team developed a novel lean-water hydrogel electrolyte through an in-situ sol–gel polymerisation process. The result is a three-dimensional hybrid inorganic–organic network that traps just enough water to keep ions mobile while locking that water in place to control reactivity.
At the molecular level, density functional theory (DFT) and Fourier-transform infrared (FTIR) analyses revealed that the hydrogel’s polar functional groups (–C=O and –NH) form strong hydrogen bonds with water molecules. These bonds reorganise water’s internal network, weakening its tendency to produce hydrogen gas.
The electrolyte uses aluminium trifluoromethanesulfonate (Al(OTF)₃) as the salt, with 1-[3-(trimethoxysilyl)propyl]urea (TU) and tetraethyl orthosilicate (TEOS) as cross-linkers, forming a stable silicon-based matrix. Even under “lean-water” conditions, aluminium ions migrate rapidly through this network, delivering both safety and conductivity.
The hydrogel electrolyte disrupts the native hydrogen-bond network of water through its abundance of polar functional groups, reducing water activity and electrolyte stability.
The resulting battery was found to have a 90% capacity retention after 200 cycles – in comparison, counterparts utilising liquid electrolytes retained only 27.8% capacity after the same number of cycles.
The open-circuit voltage also remained stable even after the battery was bent (90°) and folded (180°), highlighting the electrolyte’s excellent mechanical flexibility. Additionally, exposure to air and physical damage to the battery led to little to no reduction in performance, making the battery particularly suited for applications in wearable technology.
Rather than relying on heavy salts or costly additives to suppress water’s reactivity, the research instead showed that molecular design was a viable alternative. The discovery of an electrolyte formulation that could deliver on multiple fronts helps to bring to reality a safer, high-energy, low-cost battery alternative to current conventional lithium technology.
It is a breakthrough not just in materials but in methodology. The integration of first-principles computation, spectroscopy, and in-situ electrochemical diagnostics provided a complete picture of how atomic-scale interactions translate into battery stability. That framework now offers a template for designing other “lean-water” systems, including those based on zinc or magnesium.
This discovery also brings the promise of safer wearable technology in novel configurations, since the battery electrolyte is not only stable and safe even when exposed to air but also malleable enough to be mechanically manipulated without a performance drop.
The lean-water hydrogel’s unique balance of safety, flexibility, and ionic conductivity opens a new frontier for next-generation energy devices:
- Scalable storage: Its low-cost, non-toxic chemistry makes it a promising platform for grid-scale and residential energy storage, using Earth-abundant materials.
- Industrial translation: This discovery also opens the potential for other similar electrolyte formulations that are more stable in batteries of digering materials, expanding on potential industrial applications.
- Adaptive materials: By refining molecular interactions, future hydrogels could respond dynamically to temperature, stress, or charge — ideal for wearable electronics, biomedical sensors, and soft robotics.
Sept 2025
Spin Regulation Boosts Ammonia Oxidation Efficiency for a Cleaner Energy Future
This feature was prepared in collaboration with NTU MSE's Prof. Jason Xu and Research Fellow, Dr. Wu Qian.
Sept 2025
Spin Regulation Boosts Ammonia Oxidation Efficiency for a Cleaner Energy Future
This feature was prepared in collaboration with NTU MSE's Prof. Jason Xu and Research Fellow, Dr. Wu Qian.
Ammonia isn’t just a fertiliser — it could be tomorrow’s green fuel.
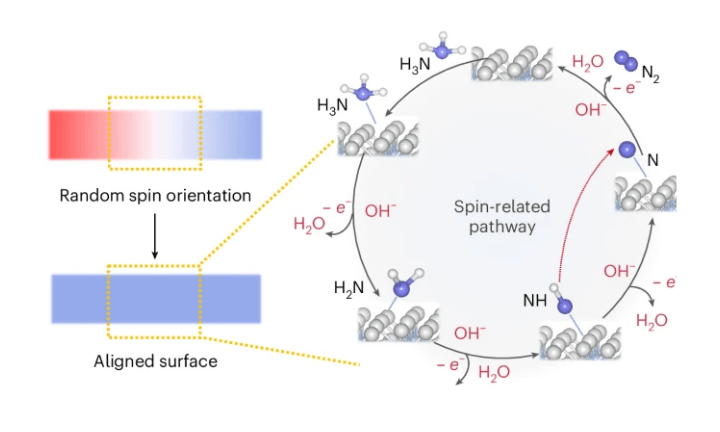
Hydrogen is one of the world’s cleanest fuels, offering an alternative to coal, oil, and gas. Ammonia has attracted growing attention as a promising hydrogen carrier, since it can be liquefied and stored in bulk under relatively mild conditions. The challenge lies in efficiently decomposing ammonia to release hydrogen. This process normally requires a lot of energy and doesn’t run smoothly. Imagine trying to play a team sport when everyone is running in different directions — that’s how the atoms behave in this reaction. Finding a way to get them “in sync” will make the process faster, less wasteful, and truly viable for clean energy.
NTU MSE researchers have shown that cooperative spin alignment can make the electrochemical ammonia oxidation reaction (AOR) more efficient — a critical step in unlocking hydrogen as a clean fuel. Using specially engineered cobalt–platinum thin-film catalysts, the team demonstrated that when nitrogen intermediates (N and NH) align their spins with the magnetic substrate, the energy barrier for their dimerisation drops dramatically, establishing a spin-regulated kinetic route to promote electrochemical ammonia decomposition.
This work introduces spin dynamics as a new design principle for electrocatalysis. Instead of relying solely on catalyst composition or structure, researchers can now consider how magnetic ordering at the atomic level influences reaction kinetics. The study not only clarifies a long-debated step in the ammonia oxidation mechanism but also establishes a precedent for integrating quantum spin effects into catalyst engineering — offering collaborators and peers with a foundation for developing next-generation catalysts for hydrogen conversion and sustainable chemical synthesis.
Building on this mechanism, the research opens pathways to:
Extend spin alignment strategies to other electrochemical reactions where intermediate coupling is a bottleneck.
Develop catalysts with tailored magnetic domains to enhance stability and reactivity for cleaner energy conversion.
Inspire interdisciplinary approaches that merge materials science, magnetism, and quantum chemistry to tackle challenges in sustainable fuels.